Top Government Food Labeling Claims
Course details
Self-Paced eLearning
199.00
2 Hours
Alton Bradshaw
This comprehensive course provides an overview of FDA and USDA food labeling claims. Participants will gain a clear understanding of the regulatory frameworks governing food labeling, including FDA nutrient content, health, and structure/function claims, as well as USDA Organic and Bioengineered labeling requirements.
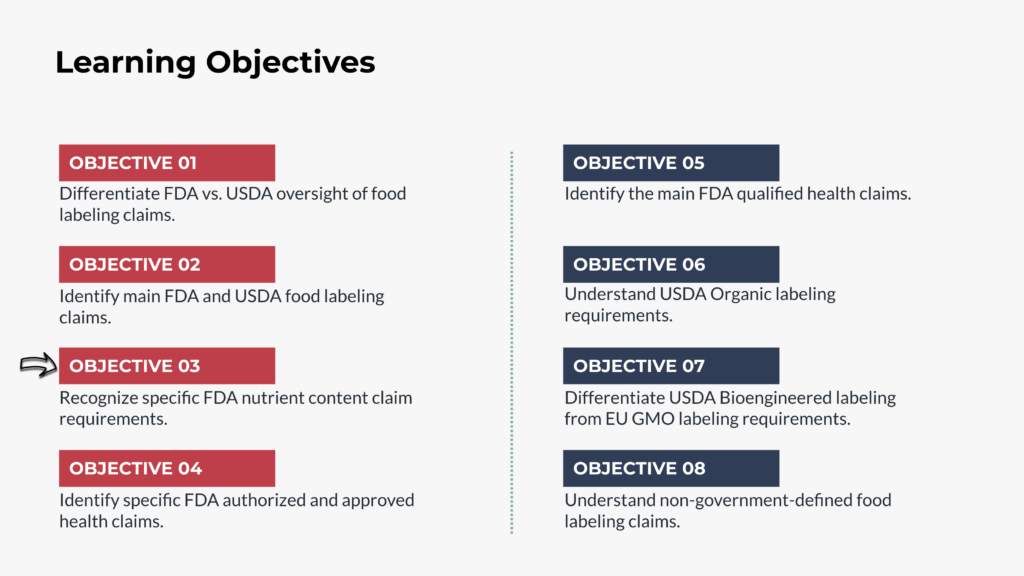
Benefits and Learning Objectives
The course will also cover important distinctions between FDA and USDA regulations, the processes for qualifying food labeling claims, and navigating government-defined and non-government-defined labeling claims.
- Understand FDA and USDA food labeling requirements
- Navigate USDA Organic and Bioengineered labeling regulations
- Review FDA claims
- Nutrient content claims
- Health claims
- Structure/function claims


Agenda:
- Comparing FDA and USDA food labeling oversight
- Overview of FDA and USDA main food labeling claims
- Reviewing specific FDA nutrient content claims
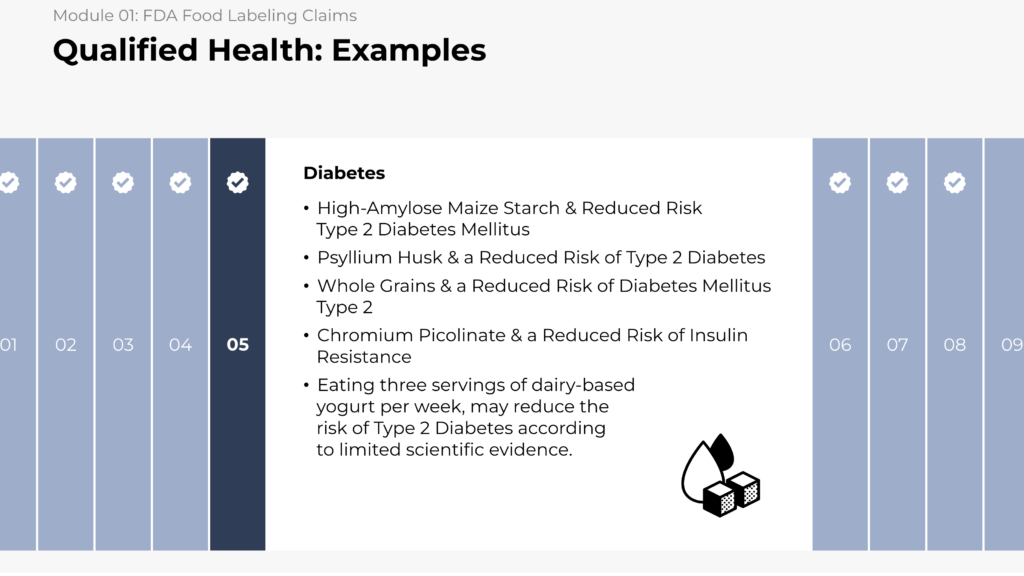
- Identifying FDA health and qualified health claims
- Understanding USDA Organic labeling requirements and processes
- Examining Bioengineered food labeling under USDA guidelines
- Contrasting USDA Bioengineered labeling with EU GMO standards
- Discussing non-government-defined food claims
Who Should Attend?
- Regulatory food labeling specialists
- Legal professionals working in food labeling compliance
- Product development scientists involved in labeling
- Quality assurance managers with food labeling responsibilities
Registration and Payment
To register for this course, please visit our training portal.

-
Vice President, Technical Services
IEH Laboratories & Consulting Group
Meet the Instructors
Alton Bradshaw
Alton Bradshaw joined IEH as Vice President of Technical Services in 2022. He holds a B.A. in Biology and a B.S. in Zoology from The University of Texas at Austin, along with B.S. degrees in Microbiology and Economics from The University of Texas at Arlington. Mr. Bradshaw also earned an M.S. in Food Safety, an M.J. in Global Food Law, and a Graduate Certificate in International Food Law from Michigan State University. Mr. Bradshaw’s MBA in Finance from the University of Texas at Arlington further adds to these achievements.
With over three decades of experience in the food industry, Mr. Bradshaw’s focus lies in food processing, including packaging of baby foods, powdered infant formula, bottled water, carbonated beverages, cereal, condiments, cookies, dairy, frozen fruits and vegetables, jams and jellies, juices, RTE salads and RTC meals, spices, and seasoning blends. His skillset encompasses a range of thermal processing techniques, from pasteurized and ultra-pasteurized to aseptic and retorted finished products, with successful regulatory resolutions with the FDA and USDA.
Mr. Bradshaw holds five professional course certifications in HACCP, Advanced HACCP, and the IDFA HACCP Individual Certification Program. He has completed Preventive Controls Qualified Individual (PCQI) and Lead Instructor Preventive Controls Qualified Individual (PCQI) training, in addition to training from Better Process Control School (BPCS) and the GMA Thermal Processing Professional Training Program (TPPTP). His vast experience includes extensive plant auditing, and he’s recognized as a Global Food Safety Initiative (GFSI) expert, completing SQF Practitioner and Auditor training.
Mr. Bradshaw is a food labeling subject matter expert with training from ESHA Research, covering Genesis R&D Software, GMA Food Labeling, Michigan State University U.S. Food Labeling, and Leatherhead Essential Guide to U.K. Food Labeling.
Over the past two decades, Mr. Bradshaw has held leadership positions at B&G Foods, Dean Foods, Hans Kissle, and Weetabix North America, overseeing food safety, quality, regulatory affairs, R&D product development, and commercialization.

-
Executive Vice President
IEH Laboratories & Consulting Group
Carol Cave
Ms. Carol Cave joined IEH as the Executive Vice President in 2024, bringing with her extensive expertise in regulatory affairs and consumer protection. She holds a B.S. in Consumer Economics from the University of Maryland.
Before joining IEH, Ms. Cave served as the Acting Associate Commissioner (ACRA) for the Office of Regulatory Affairs (ORA) at the Food and Drug Administration. In this role, she oversaw approximately 5000 ORA employees stationed across the U.S. and around the world.
Prior to her role as Acting ACRA, Ms. Cave served as Deputy Associate Commissioner, where she managed inspections, compliance, enforcement, field laboratory operations, import operations, and strategic planning.
Earlier in her career at the FDA, she served as the Assistant Commissioner of Import Operations, where she led the Office of Enforcement and Import Operations, ensuring FDA-regulated imported products comply with U.S. laws. Ms. Cave also led coordination efforts between the FDA and U.S. Customs and Border Protection, developing joint regulations, policies, procedures, and operations.
Before her tenure at the FDA, Ms. Cave was the Deputy Director of the Office of Compliance and Field Operations at the U.S. Consumer Product Safety Commission (CPSC). In this role, she collaborated with federal government agencies to identify potentially defective consumer products. With nearly 26 years at the CPSC, Ms. Cave held various leadership positions, including Director of State and Local Programs and Assistant Executive Director for the Office of Import Surveillance, ensuring the safety of consumer products nationwide.